When applying a suitable trigger, a low molecular weight gelator molecules self-assemble to form 1D structures and onto 3D networks. Like with pH-triggered gelation we can use an indirect electrochemical approach to induce a pH change in a gelator solution to made gels on an electrode surface.
low pH
If a molecule can gel at a low pH (e.g. 2NapVG) then gelation can be induced by the electrochemical oxidation of hydroquinone to benzoquinone. This reaction generates protons at the electrode-solution interface leading to a localised drop in pH and therefore gel growth. This localised drop below the pKa of the gelator triggers gel growth on the electrode surface.
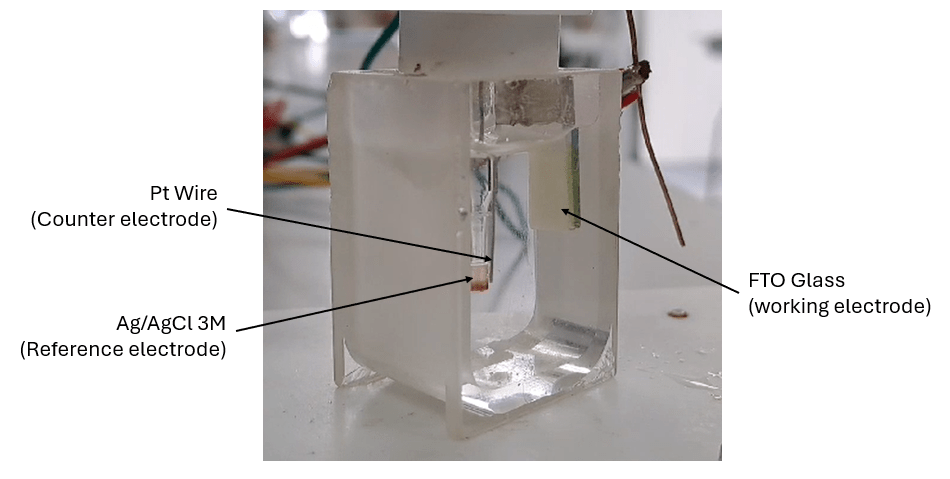
A three-electrode set-up is used consisting of a working electrode (FTO glass slide, 12 x 15 mm), reference electrode (aq. Ag/AgCl, 3 M) and counter electrode (platinum wire), in a glass beaker. To grow a hydrogels, the pH of a gelator solution (5 mg/mL) in NaCl (aq. 0.1M) is adjusted from pH 11 to pH 7. As the oxidation of HQ is accelerated at high pH, the adjustment of pH prevents the HQ in solution oxidizing before it can be consumed electrochemically. 7 mL of gelator solution containing HQ (5 mg/mL) is then pipetted into the glass beaker containing the three-electrode set-up. A galvanostatic current of current density 0.7 mA/cm2 is then applied to the working electrode (FTO slide) for 300 seconds.
For more information check out the following paper:
https://pubs.rsc.org/en/content/articlelanding/2014/mh/c3mh00150d
High pH
If a molecule can gel at a high pH (e.g. Fmoc-3) then gelation can be induced by the electrochemical reduction of hydrogen peroxide. This reaction generates hydroxide ions at the electrode-solution interface leading to a localised increase in pH and therefore gel growth. To form the hydrogel, the gelator molecules self-assemble in water to form fibrous structures that entangle when the pH of the solution is raised above the pKa .
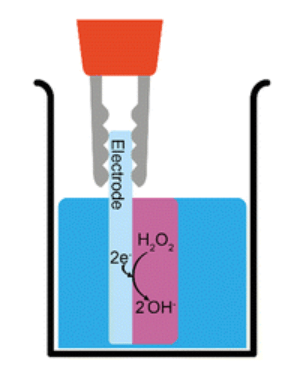
A three-electrode set-up is used consisting of a working electrode (FTO glass slide, 12 x 15 mm), reference electrode (aq. Ag/AgCl, 3 M) and counter electrode (platinum wire), in a glass beaker. To grow a hydrogel, 7 mL of a gelator solution (5 mg/mL in aq. NaCl 0.1 M) is prepared to give a gelator solution at a pH of 6.5. As carbon dioxide in the atmosphere readily dissolves in water to give dissolved CO2, carbonic acid and the bicarbonate anion, it is likely that the pH is fixed at 6.5, within the range of the apparent pKa value of this system, due to the natural buffering of these dissolved carbon dioxide species in the gelator solutions. To create the basic pH gradient at the electrode surface, 70 μL of hydrogen peroxide solution is added to the gelator solutions immediately prior to gelation. A current density of −0.7 mA cm−2 is then applied to the solution for 900 seconds.
For more information check out the following paper:
https://pubs.rsc.org/en/content/articlehtml/2023/qm/d3qm00091e