Adams-group-specific abbreviations
A great many abbreviations for functional groups are in use in chemistry. Some are ubiquitous e.g. Ph for phenyl, or Bz for benzoyl, others are more obscure and used in specialised contexts, for example ever heard of the Msib protecting group? These naming conventions are important enough to be listed in chemistry texts. However, other abbreviations are made up for the convenience of individual research groups and are not known (or worse, have different meaning) outside that group. In the Adams group there are a couple of abbreviations which only we use detailed below.

“Nap”
There is a protecting group called “Nap” (2-naphthylmethyl), but its structure is not the same as what people understand by “Nap” in the Adams group. To be clear, in the Adams universe, “Nap” stands for “2-(naphth-2-yloxy)acetyl”, not “naphthyl” or “2-naphyhylmethyl”:
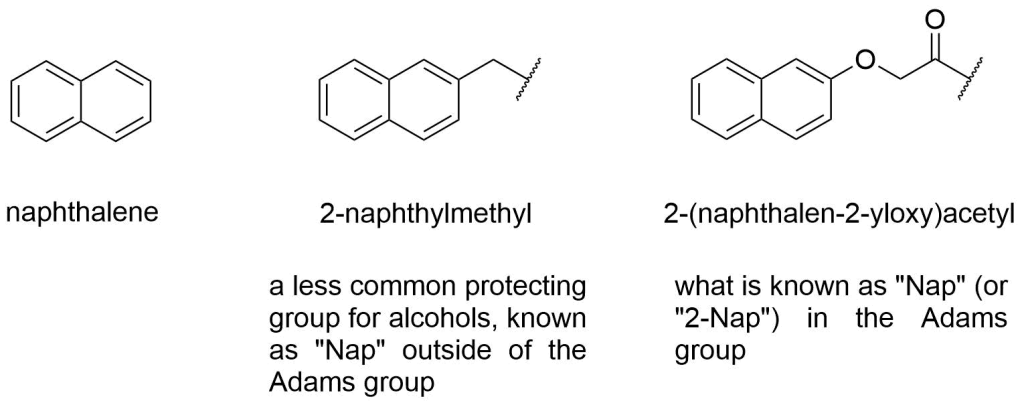
Speaking of “naphthyl”, when you’re synthesising NapFF (or other Nap-compound), make sure you grab 2-naphthoxyacetic acid from the cupboard, not 2-naphthylacetic acid they’re different compounds!

Among the possible attachment points for the acetyl bit on the naphthalene ring, the 2-position is by far in most common use in the Adams group and therefore the “2” is often omitted: “Nap” implies “2-Nap”. 2-Nap is in fact so common in the Adams group that the Nap part itself is sometimes omitted: FF can mean 2-NapFF (except where it means just FF, i.e. the dipeptide consisting of two phenylalanines: potentially confusing but usually clear from the context) and BrAV is actually 6-Br-2-NapAV. Be aware however that some gelators are derived from 1-naphthol and therefore contain “1-Nap” in their name. The attachment position is important: 1-NapFF and 2-NapFF are two completely different compounds! Additional substituents on the naphthalene are prefixed to the Nap part, including their positional index (as in 6-Br-2-NapAV).

Modified “Nap”
Some gelators contain a modified naphthalene core where half of the ring system has been hydrogenated. This core is called 5,6,7,8-tetrahydronaphthalene and the building block (including the acetyl bit) is abbreviated “1-ThNap” in the Adams universe. Note that the “1-” is sometimes left out: “ThNap” implies “1-ThNap”. Note that this is different from “Nap” which implies “2-Nap”! Not very consistent but that’s just how it is.


Amino acids and prefix/suffix notation
One- and Three- letter amino acid abbreviations
Protecting group abbreviations
By convention, any additional text to the left of the amino acid abbreviation is associated with its N-terminus, and any text to the right of the abbreviation with the C-terminus. So, for example, BocPhe is N-Boc-protected phenylalanine and PheOEt is phenylalanine ethyl ester. BocPheOEt would be N-Boc phenylalanine ethyl ester.
Amino acids with free amino groups and protected carboxyl groups (i.e. esters) often present as waxy solids or sticky and viscous oils which are a pain to handle in this form. They are therefore usually sold as their hydrochloride salts (less often salts of other acids) which present as easy to handle white powders. Another reason for keeping amino groups in the form of salts is to prevent air-oxidation and reaction with atmospheric CO2. In keeping with the left-right convention, the acid should figure on the left of the abbreviated name, but is often found on the right. It is separated from the amino acid part of the name by an interpunct (midline dot or multiplication dot). So, TFA.Phe and Phe.TFA both mean the same: phenylalanine trifluoroacetate.

When an amino acid abbreviation has no prefix or suffix, this indicates that the respective terminus is not substituted (i.e. free amine or carboxylic acid). Sometimes, the fact that a terminus is free (unsubstituted) is emphasised by an explicit hydrogen (H) for the amino group (left side) or explicit hydroxyl (OH) for the carboxyl group (right side). Hyphens sometimes separate the amino acid abbreviation from the explicit H or OH. So, Phe, H-Phe, PheOH, and H-Phe-OH all represent phenylalanine. The same/similar conventions are used for peptides and even non-amino acids (e.g. the Nap building block).

To be clear, the correspondence of N-terminus to prefix (left) and C-terminus to suffix (right) holds no matter in what orientation the compound is drawn! In all the above, the amino acids were drawn with N-terminus on the left and C-terminus on the right (mirroring the prefix/suffix convention) but a chemical structure can be drawn in any of many orientations. The prefix/suffix convention does not change:


Amino acid stereochemistry
All amino acids (apart from glycine) come in two enantiomeric forms: the naturally predominant L-enantiomer and the opposite D-enantiomer (found in some exotic critters and bacterial cell walls). The L-amino acids are ubiquitous, much cheaper than their D-isomers, and far more widely used. Most of our gelators contain the L-isomers. If a gelator does not specify its stereochemistry, you can assume it’s L. The stereochemistry is L unless otherwise noted.
The older D/L system of classifying the configuration of amino acids is based on the comparison of their spatial configuration to that of glyceraldehyde. The dextrorotatory (rotates polarised light clockwise) form of glyceraldehyde was arbitrarily assigned the label “D” and amino acids which can (in theory) be derived from it also carry that label. The other enantiomers are “L“. A more recent system (the R/S system) is based on the atomic weights of atoms attached to the stereocentre. Long story short, most L-amino acids are (S)-amino acids and conversely, most D-amino acids are (R)-amino acids. The exceptions are cysteine and selenocysteine, where the “natural” L-cysteine (L-selenocysteine) is the (R)-isomer in the R/S system. For amino acids: L is (S) and D is (R), except for cysteine and selenocysteine. In the Adams group, we tend to use the R/S system but you should be aware of both.
We do use some gelators with D-amino acids for specialised applications. These will be explicitly labelled with their stereochemistry, e.g. (RS)-2NapFF, or 2Nap(R)F(S)F. If you’re looking for standard NapFF and find a vial labelled (SR)-NapFF, do not use it, it’s not what you’re looking for!
Similarly, amino acids from the cupboard will also be explicitly labelled with their stereochemistry, particularly if it’s not the “natural” one. So, if you’re looking for standard phenylalanine (i.e. L-phenylalanine, the “natural” isomer) and find a small bottle labelled D-phenylalanine, it’s not what you want, keep looking! The “natural” phenylalanine will either be explicitly labelled (L-phenylalanine or (S)-phenylalanine), or have no stereochemical label (in which case the default is L).
Amino acids that are 50:50 mixtures of their (S) and (R) isomers (racemates) are occasionally useful. These are also explicitly labelled as such, e.g. DL-phenylalanine or rac-phenylalanine. So if you’re looking for standard phenylalanine and find a bottle labelled DL-phenylalanine, keep looking!
