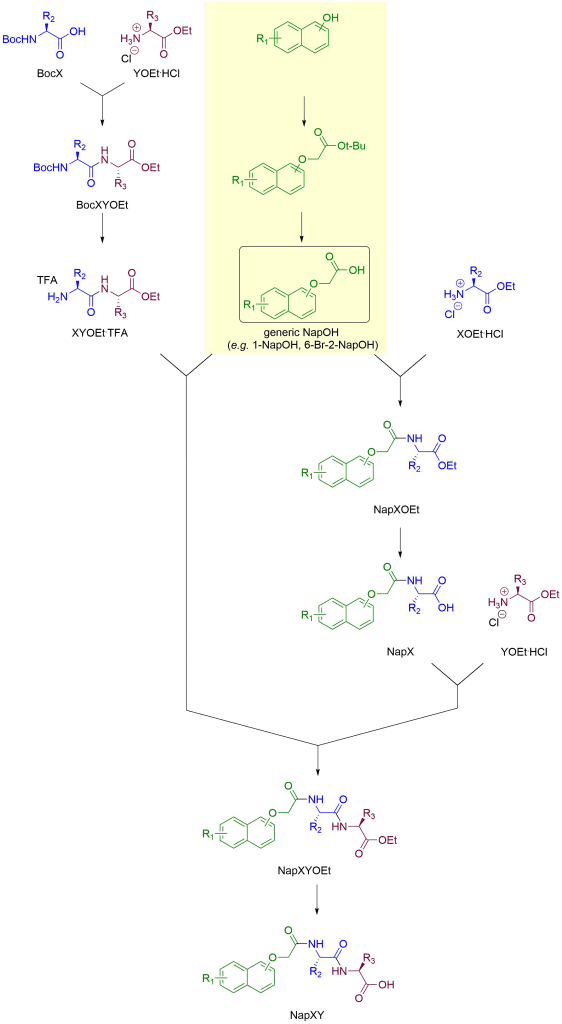
In the following, structures are deliberately kept generic. The hydroxy substituent and optional side group (R1) on the naphthalene-based building block are not in fixed positions. Generic amino acids are denoted by R2 and R3 side groups in their structures and X and Y in labels. The C-terminus of the amino acids is usually protected as an ester: ethyl esters are shown on this page but methyl esters are also commonly employed. Make sure your mass calculations employ the ester you are actually using!
One of the approaches on this page is applicable to the synthesis of any of our Nap-dipeptide gelators, and probably beyond. Most gelators could probably be made by either approach. Generally, if valine is involved, we use the “stepwise route”, otherwise the “dipeptide-first” route. This is down to the fact that, once upon a time, Dave tried the “dipeptide-first” route for some gelators containing valine and found the workup of one of the intermediates messy. Or the yield low. Or something. This is all folklore now but that’s how we do things. If you do make a valine-containing gelator via the “dipeptide-first” route and do not encounter any issues, do let us know!
“Dipeptide-first” route
Most commonly, we assemble the dipeptide first, then attach it to the Nap building block. This route is highlighted in yellow below. We use iso-butyl chloroformate/N-methylmorpholine in chloroform for the coupling of the amino acids and then the coupling of the dipeptide to the Nap building block. The reasons for this particular choice of coupling reagents (among the myriad peptide coupling protocols available) are lost in deep geologic time, but probably have to do with ease of workup, low cost, and “working well enough”. If you do find a better route (better in at least one aspect, equal or better in all others) please do let us know!
The removal of the Boc group is done by trifluoroacetic acid in chloroform while the ethyl ester is removed by lithium hydroxide in aqueous tetrahydrofuran solution. For more observations and tips on these deprotections, see here. The synthetic pathway comprises 4 steps (assuming the NapOH building block is commercially available).
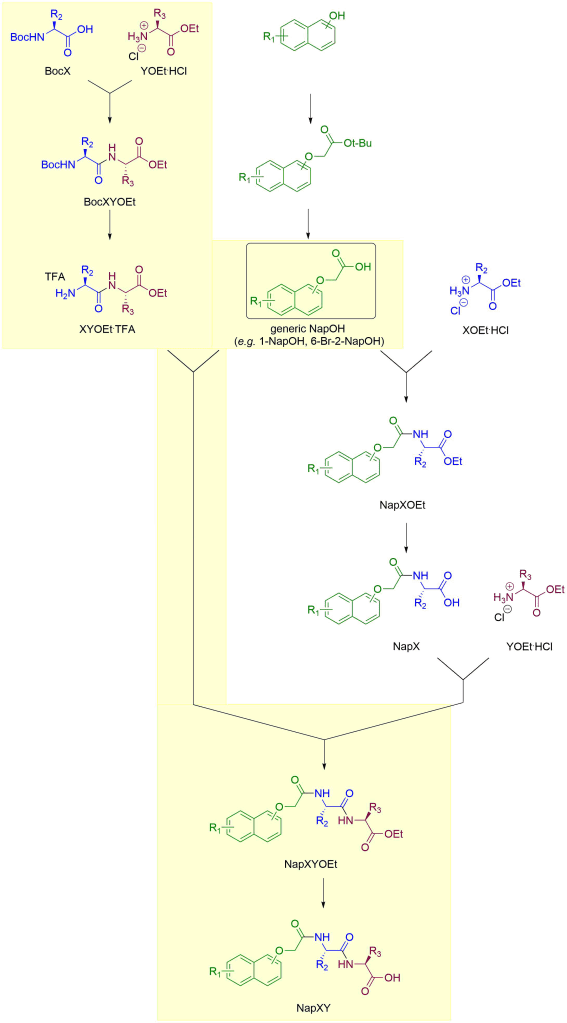
“Stepwise” route
For gelators containing valine, the stepwise route is preferred. It is highlighted in yellow below. Here, rather than assembling the dipeptide first and then attaching it to the Nap building block, we attach and deprotect the individual amino acids to the Nap building block one at a time, much as a peptide synthesiser robot would do. The coupling and ester deprotection reagents are the same as for the “dipeptide-first” route. Note that no Boc protecting goups are involved in this approach. Assuming the NapOH building block is commercially available, this route also takes 4 steps.
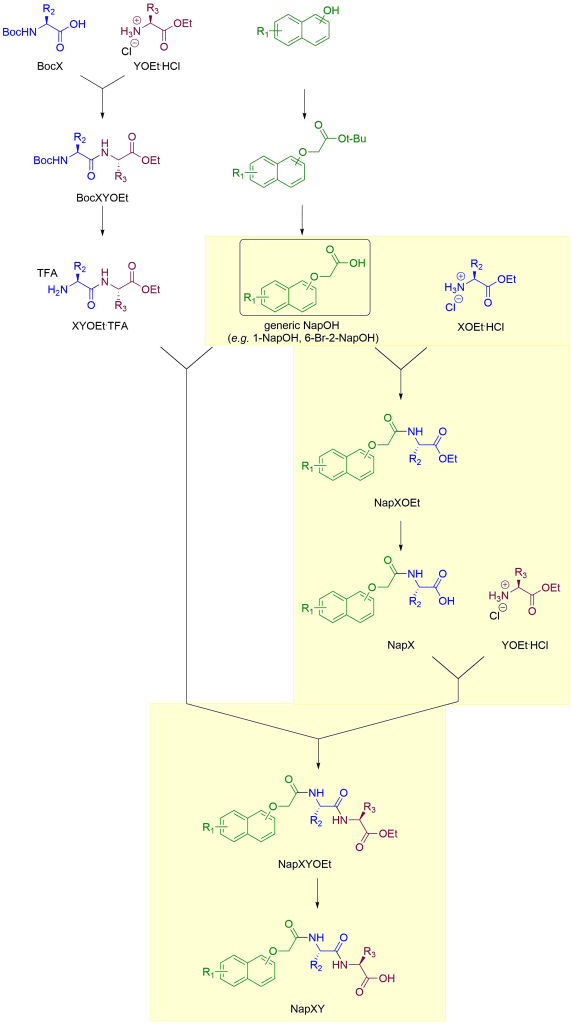
The Nap building block
The naphthalene-containing building block NapOH (see naming) is available commercially only for simple (i.e. otherwise unsubstituted, R1=H in the schemes on this page) naphthalenes. So that means only 2-NapOH (2-naphthoxyacetic acid, CAS 120-23-0) and 1-NapOH (1-naphthoxyacetic acid, CAS 2976-75-2) are commercially available (at least at sensible prices). All other (R1≠H) NapOH building blocks have to be synthesised from the appropriately substituted naphthols. This adds two steps and is highlighted in yellow below.