Altering the pH of a micellar solution is an effective means of controlling hydrogelation as very small amounts of acid or base can introduce significant changes in pH.[1] This method relies on the presence of a functional group within the LMWG which can be reversibly protonated or deprotonated, such as a carboxylic acid or amine (Figure 1).
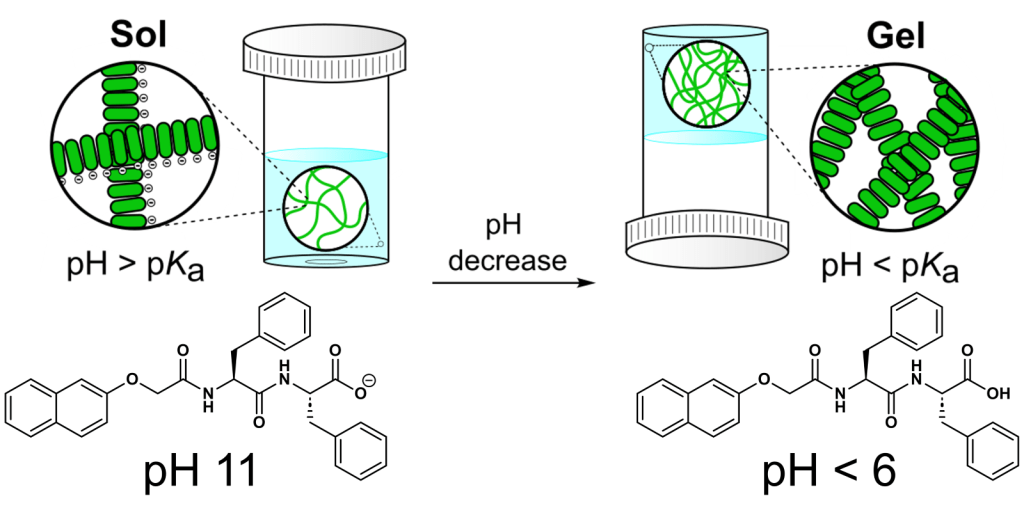
Figure 1: Scheme illustrating the pH-triggered gelation of 2NapFF, a commonly used gelator molecule for the Adams group.
While changing the pH will influence the strength of hydrogen-bonding interactions between the LMWG and water molecules, the predominant advantage of this trigger is that the solubility of the gelator can be modified depending on the pH of the environment.[2] Generally, for gelators containing acidic groups, dissolution first takes place at high pH so to cause deprotonation of the functional group, thereby maximising charge-dipole interactions. The solubility of the LMWG is then decreased by lowering the pH below the apparent pKa of the gelator, thus causing re-protonation and triggering the self-assembly process.[3] This is a diffusion-limited process, and so while acids such as aqueous hydrochloric acid (HCl) can be used, this can lead to the formation of inhomogeneous gels.[4] This occurs when the rate of protonation, and therefore gelation, is faster than the rate of HCl diffusion through the sample.[5] In order to form homogenous hydrogels, controlled acidity can be introduced via the addition of glucono-δ-lactone (GdL).[4] GdL is a sugar, and hydrolyses in water to release protons, which can readily re-protonate the gelator. Through this method, the rate of hydrolysis is slower than the rate of GdL diffusion in the solution, allowing for a uniform distribution of protons and resulting in homogeneous gels (Figure 2).[4] Other substrates such as methyl formate can also be used.
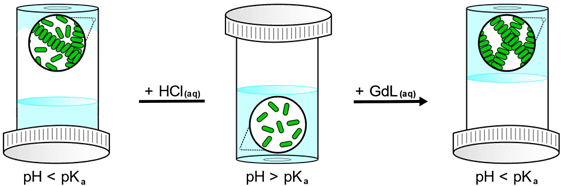
Figure 2: Scheme comparing pH-triggered gelation via the addition of HCl(aq) and GdL(aq).
Preparation of Micellar Solutions
Aqueous stock solutions of LMWG are prepared by suspending the gelator in deionised water, followed by the addition of 0.1 M (1 molar equiv.) NaOH(aq) to give a solution of known concentration. Self-assembly processes within these systems can be very sensitive, and varying factors such as stirring rate can result in the formation of different nanostructures. Therefore it is important to follow a consistent protocol when preparing new batches. Typically, within the Adams group, suspensions in 50 mL Falcon tubes wrapped in Parafilm are stirred using 25 x 8 mm stirrer bars at 1000 rpm. Care should be taken to ensure all solid residues are dislodged from the side of the Falcon tube before leaving overnight. 16 hours later, the micellar solution is pH adjusted to the desired pH. This is dependent on the identity of the gelator, and the structures that are formed. For example, micellar solutions of 2NapFF are typically pH adjusted to pH 11, as this is above all apparent pKa values. To increase the pH, small (typically ~5 μL) aliquots of 1 M NaOH(aq) is added. To decrease the pH, 1 M HCl(aq) is added, or the solution is allowed to stir in air.
The pH of Fmoc-containing gelator solutions should not be adjusted to a value greater than pH 10.5, as Fmoc is a base-sensitive protecting group. All solutions should be prepared freshly, especially Fmoc gelator based solutions.
Preparation of pH-triggered gels
To form a pH-triggered gel, a given volume (typically ≥ 2 mL) of pH-adjusted micellar solution is pipetted into a vial containing pre-weighed GdL. Generally, 5 mg/mL gelator solutions contain 8 mg/mL GdL, and 10 mg/mL gelator solutions contain 16 mg/mL GdL. Upon addition of the solution, the vial is thoroughly swirled by hand for 5 seconds, before placing the vial down to allow for gelation. The sample should then be left overnight. Care should be taken to not disturb the sample so not to disrupt any self-assembly processes. Gelation kinetics are affected by LMWG structure, concentration, temperature and pH, so different conditions may afford different gelation timescales. Again, 16 hours is a good starting point. Overnight pH measurements are a useful way of determining how long it takes for the pH of these systems to plateau.

Figure 3: 16 hour pH log for a system containing GdL
Organic solvents, such as methyl formate is also an effective substrate which can be used to gradually decrease the pH of a system, at a slower rate than GdL will. Ethyl formate and propyl formate will decrease the pH of a system at even slower rates. Here, the pH-adjusted micellar solution is first pipetted into a vial, followed by pipetting the methyl formate within the solution. Methyl formate is highly volatile, and so the process of pipetting up and transferring to the solution should be completed as fast as possible. This should also be completed in a fumehood. The sample should then be swirled by hand for approximately 5 seconds before allowing to sit overnight. Again, it is optimal to run an overnight pH measurement to ascertain appropriate timescales.
[1] T. Kar, S. Debnath, D. Das, A. Shome, P. K. Das, Langmuir 2009, 25, 8639–8648.
[2] X. Du, J. Zhou, J. Shi, B. Xu, Chem Rev 2015, 115, 13165–13307.
[3] C. Tang, A. M. Smith, R. F. Collins, R. V. Ulijn, A. Saiani, Langmuir 2009, 25, 9447–9453.
[4] D. J. Adams, M. F. Butler, W. J. Frith, M. Kirkland, L. Mullen, P. Sanderson, Soft Matter 2009, 5, 1856–1862.
[5] Z. Yang, G. Liang, M. Ma, Y. Gao, B. Xu, J Mater Chem 2007, 17, 850–854.