How to prepare transient gels
Supramolecular materials that are responsive to pH changes can be designed such that they transit through different phase changes (sol-to-gel-to-sol, gel-to-sol-to-gel, gel-to-gel-to-gel). The phase changes will rely on system design and molecular structure. Generally, a transient process can be achieved by implementing different pH triggers within the sample, leading to an increase and subsequent decrease in pH or vice versa. The lifetime of each phase will rely on concentration of gelator molecule and pH triggers, which allows a high level of temporal control over the evolution of the systems.
As an exemplary system, we show here the preparation of a gel undergoing a gel-to-sol-to-gel transition based on gelator 1ThNapFF. For further explanation of the system, the reader is referred to the following publications: https://doi.org/10.1021/acs.chemmater.0c01483, https://doi.org/10.3390/gels8020132.
Formation of the initial gel relies on solvent-triggered gelation, achieved by diluting a high concentration of gelator solution in an organic solvent (e.g. DMSO, EtOH) with H2O. The first step in the preparation is then to prepare the high concentration solution of the gelator. The concentration of this solution will depend on desired final quantity in the material and solvent ratio within the final gel. To prepare a 2 mL gel of 2 mg/mL 1ThNapFF in DMSO/H2O, 20/80 (v/v), 0.4 mL of DMSO of the initial solution will be used. Hence, a 10 mg/mL solution of 1ThNapFF will be prepared in DMSO. The gelators are readily soluble in DMSO, but gentle heating can be used to aid dissolution if required at higher concentrations.
0.4 mL of this solution will then be transferred to a 7 mL Sterilin vial (Figure Xa). Here, the pH triggers will be introduced prior to gelation to ensure that the transient system operates autonomously. The competing pH triggers added to this exemplary system will be urea and urease to obtain fast pH increase and methyl formate to induce a slow pH decrease. It is worth noting here that different triggers can be used for such systems, depending on the ideal timescales of evolution (https://doi.org/10.1021/nl5039506). To 0.4 mL of gelator, a small quantity of urea in H2O will be added, depending on the required final concentration of the trigger. For a final concentration of 0.02 M, 20 μL of 2M urea will be transferred to the vial and briefly swirled to avoid any local gelation due to addition of H2O (Figure Xb, left vial). At this step, it is very important to ensure that no gel clumps are formed (Figure Xb, right vial).
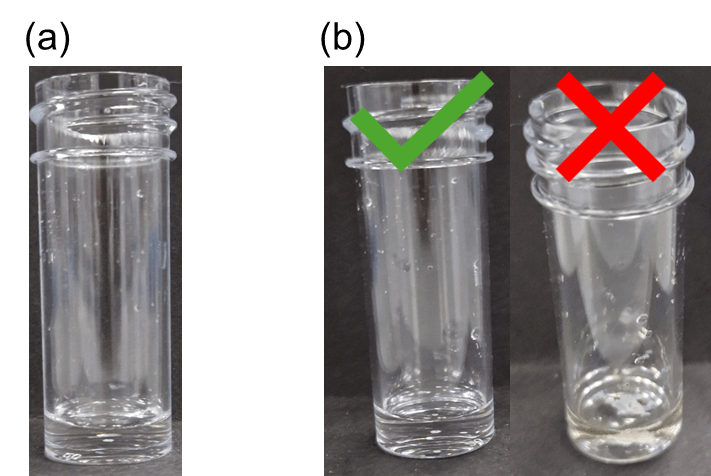
Figure 1. (a) Photograph of a 7mL sterilin vial containing 0.4 mL of a 10 mg/mL 1ThNapFF solution, (b) photographs of vials containing 0.4 mg/mL of 10 mg/mL 1ThNapFF and 0.02 μL of 2M urea. The left vial was briefly swirled after addition of urea, while the right vial was not. Localised gelation can be observed in the vial on the right.
To this, methyl formate will be added (100 μL in the material shown) and gently swirled. Finally, to form the gel, a solution of urease in H2O will be added in one aliquot to reach a final volume of 2 mL. For a final concentration of 0.2 mg/mL, 1.580 mL of 0.253 mg/mL urease in H2O will be added to the system. A gel will immediately form after this, so it is essential to pipette the solution in one aliquot (Figure X, left vial). It is also important to ensure that the aqueous solution is not added too fast to avoid formation of bubbles in the gel (Figure X, right vial).
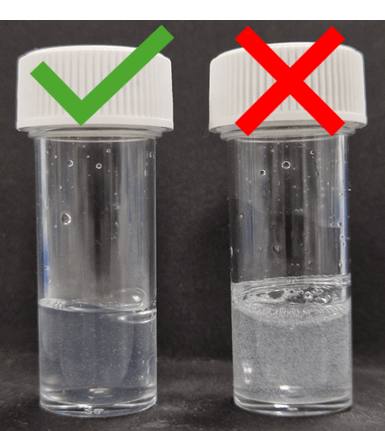
Figure 2. Photographs of gels formed after addition of urease aq. solution to a final volume of 2mL. Urease was pipetted in one aliquot in both samples, but with different speeds. The sample on the right shows a gel obtained with fast pipetting, with formation of a significant number of bubbles.
Once the gel is formed, the gel-to-sol-to-gel transition will occur, with timescales dependent on the concentrations of urea, urease and methyl formate. In this case, the gel-to-sol transition occurs within 8 minutes and re-gelation takes place after 6 hours. Typically, the samples are left undisturbed for 16 hours to ensure that the pH cycle goes to completion (Figure X).

Figure 3. Photographs highlighting the gel-to-sol-to-gel transitions occurring in the system prepared in this section.